International Childhood Cancer Day happens every year on 15 February. This year, we caught up with Little Princess Trust-funded researcher Dr Rhys Morgan.
Rhys is an Associate Professor of Cancer Biology at the University of Sussex and leads a research team that focuses on acute myeloid leukaemia (AML). His younger brother, David, died from relapsed blood cancer at age 21, after being diagnosed as a teenager. David is just one of the reasons why Rhys is so committed to his work. Here’s what he had to say…

Dr Rhys Morgan
The theme of International Childhood Cancer Day this year is ‘Inspiring Action’ – why do children and young people with cancer need action and change?
Firstly, you have different considerations with children and young people, because of their age. There are long-term impacts on fertility, education, and income, which perhaps aren't such big considerations for older people with cancer.
We also know that children's cancers are different genetically, metabolically, and molecularly to adult cancers. There's a medical need to treat these two age groups differently, but at the moment we are mostly just using the same treatments used in adults. These are not really effective for children, so change is needed to develop age-specific targeted treatments.
The toxicity of these treatments really stays with you. I've got a lasting memory from when my brother was undergoing blood cancer treatment. Me and my family were in David’s room on the ward watching Wales versus England, which is quite a big thing for us. I just remember him vomiting the whole way through it. I don't remember the game or the score even because all I could see in my peripheral vision was David really struggling. That inspires you to think there's got to be a better way than this.
It must be very difficult seeing a loved one go through that. What was David like?
I was a couple of years older than David. He was quiet, unassuming, and very smart. He liked what other people his age liked, such as sports and gaming – and girls. But he was not one to follow the crowd. He liked his quiet space and was a gentle giant.

Rhys' brother David.
He first got cancer when he was 17 and then he went into remission for a few years before it came back at the age of 20. At that point, it was drug-resistant, ultra-aggressive and untreatable. But I think after that first diagnosis, David became more philosophical in a way. He was enrolled to do a law degree at University West of England, but unfortunately, he didn't make it that far.
I imagine getting diagnosed with cancer at such a young age could definitely have a big impact on that.
I guess it changes your world perspective of everything. When David was first diagnosed, he didn't think it would eventually end his life. It wasn’t terminal to start with and he did go into remission for a few years. But I think, during that time, he was thinking more about the world and his place in it.
Did you want to do research before David’s diagnosis, or were you already interested in science?
I did an undergraduate biomedical science degree, which covers all aspects of biology and medicine. I think David was first diagnosed when I was in my first year and I was already starting to get very drawn to haematology – the study of blood.
I think David’s diagnosis reinforced my plan, given that he had blood cancer. In my first year of the degree, we didn't really get an insight into research at all. But I did my final year research project and realised I could make a career out of this - using my skills and applying them to a problem I've experienced.
It is clear that David’s experiences have had a lasting impact on you. As a researcher, and in general life, how has he inspired you?
There are two ways that David has inspired me.
The first is the horror of seeing a family member go through something like that, the treatments they had and then how sick they were. It inspires you to help prevent that in the future.
Then there's the other aspect to it, which is the bravery and the courage. Even though David was going through all that, he was always keen to power through. On days when I'm in a low mood or feeling a bit demotivated, when I think back to his battle, it makes me want to get a grip and kick on if I can because he went through a lot worse.
I also donated my bone marrow stem cells in 2016 to an anonymous recipient who is doing well to this day. I was keen to get the opportunity to do it because the next line of treatment for David was going to be an allogeneic stem cell transplant. They use a matched donor for this, like a family member, and your healthy donation basically replaces their existing diseased bone marrow.
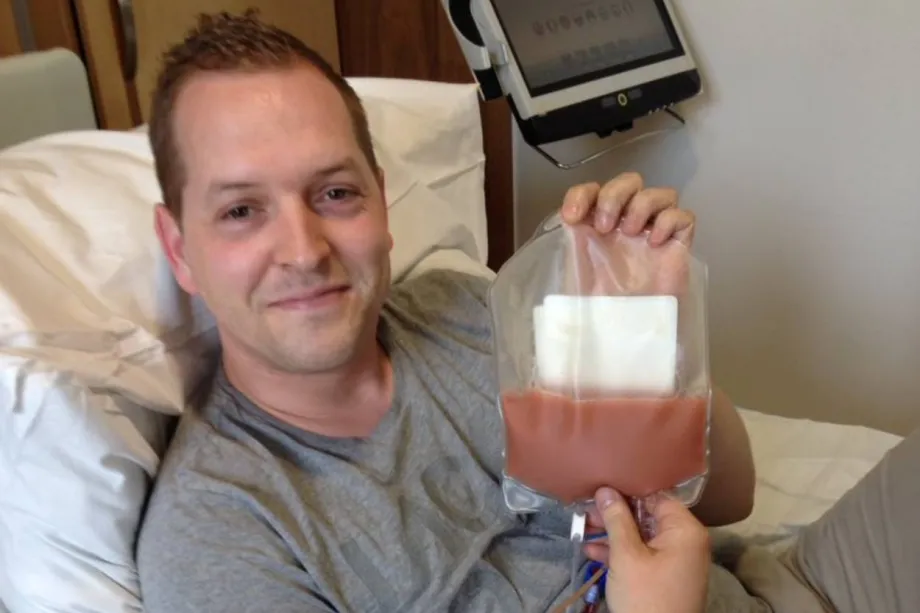
Rhys and his stem cells.
I was the tissue match for David, but his disease came back so aggressive and so drug resistant that we couldn’t even get to the point of attempting the procedure. It was really nice to be able to do it for someone. Just, unfortunately, it wasn't my brother.
It is an amazing thing to be able to help someone – what was the donation like?
There are a lot of myths about donating bone marrow stem cells - everyone says that it must be painful, or that you have to drill into the bones to get it but that's just not true. You're given a course of injections for four days (G-CSF) to mobilise the stem cells into the blood. Then they can be simply drawn off like a regular blood donation.
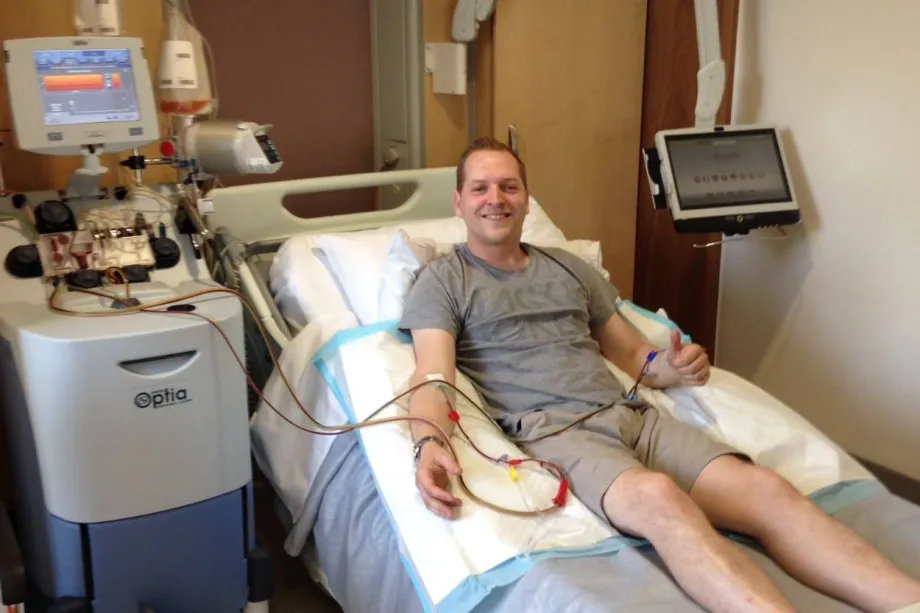
Rhys donating stem cells.
You can sign up through Anthony Nolan – you just complete a form and send them some cheek swabs. Then, and only if they find a match, you get invited for the donation and they make everything as convenient and easy as possible.
When I teach students, I try to get as many signed up as possible because I don't think they realise how easy it is. Because patients need stem cells from someone who is very like them, so that their bodies don’t reject the transplant, it can be difficult to find matches. On campus, you have all the demographics, like ages and the mix of different heritages, which is really difficult to match tissue for. I always try and use the opportunity to get lots of people signed up.
Not only are you potentially helping people going through treatment, but you are also researching childhood leukaemia. Can you tell me a bit about what you are doing?
My lab is working on something we’ve known about for a while, but we’re taking a slightly different angle. I’ve worked on a molecule called beta-catenin since my PhD, which has historically been a real problem in the cancer field. It's mutated in a lot of solid tumours and it’s overactive in leukaemias, particularly childhood leukaemias. We've known about it for decades, but there's currently no effective way to target this molecule.
It's well known that beta-catenin is involved in transcription inside cancer cells, which means it’s a part of reading DNA and activating genes. A lot of the attempts to target beta-catenin have been focusing on that first step, gene activation, and it's not really worked. But there's a whole complicated ‘post-transcription’ process after activating the genes, where the cell turns that gene into a functional molecule. We found that beta-catenin is very involved with post-transcription.
My interest now is whether we can target beta-catenin's activity in post-transcription therapeutically and if it reduces the growth or survival of leukaemia cells.
We are getting more interested in post-transcriptional genes generally as it seems to be a very important but much-understudied facet of leukaemia biology. We have other projects assessing post-transcription in the presence of two other frequently occurring mutations in acute myeloid leukaemia; WT1 and RUNX1.
I have a great team with a fantastic blend of scientists and clinicians who are bringing the new expertise and fresh enthusiasm we need to achieve our lab goals. It’s an exciting time.
What impact do you think your work could have?
If we can understand the biology of what beta-catenin is doing at that stage, the theory is that we'll be able to design something to disrupt that activity.
What we all want in cancer is targeted treatments, not this blanket chemotherapy. So, if the data starts to show that beta-catenin is involved in a process that helps leukaemia cells grow, helps them survive or become resistant to therapy, then maybe researchers can start to design a treatment that specifically targets that process.

Rhys’ lab group at the University of Sussex.
We're very much in the discovery phase at the moment. We’re trying to understand how these molecules work and how they contribute to leukaemia development. I think the next stage, if successful, is to team up with medicinal chemists and find molecules or inhibitors that might disrupt beta-catenin’s activity. If that looks promising, then we’d switch to preclinical models to assess safety, ahead of any clinical trials involving patients.
The work we're doing now is at least a decade off being used in the clinic, but you can't get there without undertaking this kind of rigorous groundwork testing first.
Is there anything you'd like to tell parents and charities about your work?
Research can’t happen without funding, and the number of funders for this type of work is becoming vanishingly small. I've not managed to obtain government-level funding, so all of the money for my research has come from charities.
We simply wouldn't be doing our research now if it wasn't for blood cancer charities or charities like the Little Princess Trust and CCLG.
We get a little bit from the university department here and there, but it's not enough to do the type of ground-breaking science that young people with cancer need.
I just want to tell people that you may think your donation is not a big one, but anything really, really does help.

Ellie Ellicott is CCLG’s Research Communication Executive.
She is using her lifelong fascination with science to share the world of childhood cancer research with CCLG’s fantastic supporters. You can find Ellie on X: @EllieW_CCLG




