In the CCLG research team, we talk a lot about targeted treatments that use drugs to stop cancer cells from growing and spreading. It is the foundation of precision medicine and is based on learning more about the DNA changes that drive cancer, so that we can design better treatments to target these changes without damaging healthy cells. Here, we explain what this means.
To understand how targeted therapies work, we first need to understand the difference between a healthy cell and a cancer cell. Our entire body is made up of cells, and each cell is specialised for a purpose. For example, red blood cells carry oxygen through our blood while nerve cells are part of our central nervous system and can transmit feelings of pain to our brain. These variations are controlled by a cell’s genetic code. This code acts like a set of instructions for a cell, telling it what job it must do, how often it should multiply, and much more.
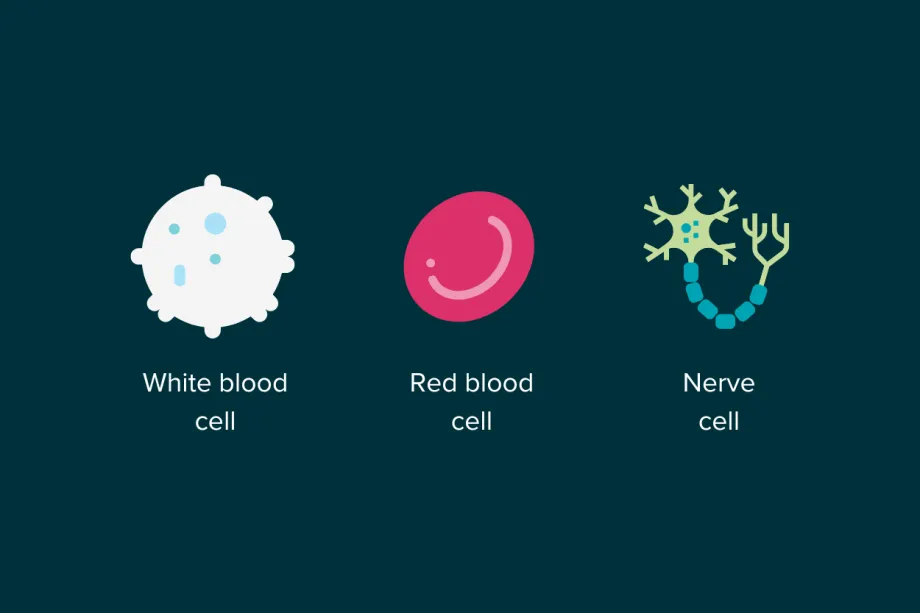
Different types of cells can look and behave very differently, but all of this variation is controlled by the genetic code.
What makes cancer cells different?
It's easy to kill cancer cells outside of the body, such as in a lab, because you don’t have to worry about also killing healthy body cells. Cancer treatment dosage may need to be lower for patients as cancer treatments often harm healthy body cells. This is because cancer starts from a normal healthy cell that has mutated DNA. This mutated cell then starts to multiply out of control and can become cancerous. These new cancer cells are different to healthy cells because of the damaged DNA - such as being a different size or shape, having a genetic code that doesn’t arrange itself properly, and losing distinct boundaries between cells. However, because they trace their origins back to normal body cells, they still have a lot in common with healthy cells.
Researchers look at healthy cells and cancer cells to find something different in or around the cancer cell that is helping it to grow and spread. This is a potential ‘drug target’. For example, a cancer cell may need a specific protein to survive, so removing the supply of that protein could kill that cancer cell. From this information, researchers can then design targeted treatments such as a drug or combination of drugs to use in patients.
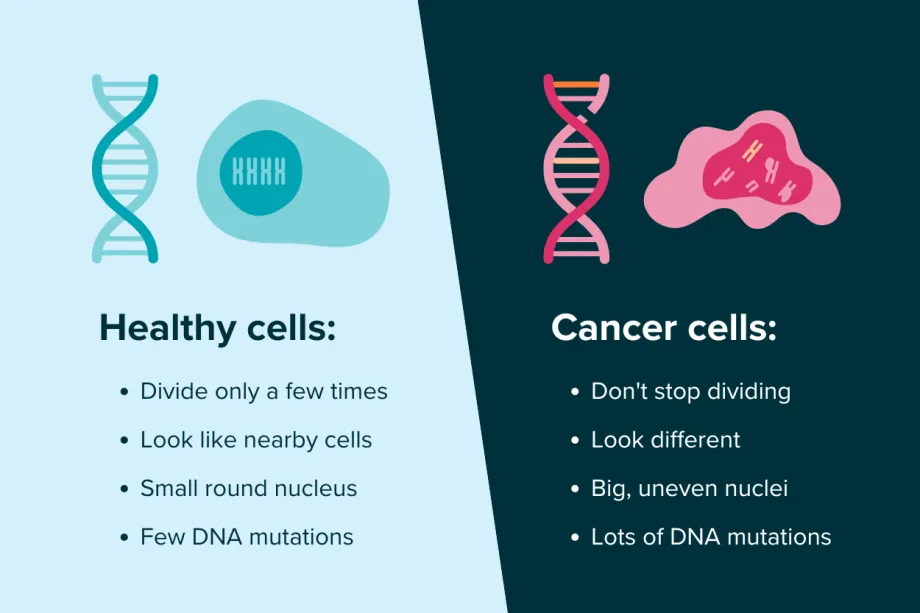
The differences between healthy cells and cancer cells.
Discovering drug targets in lymphoma cells
Researcher Professor Vikki Rand is trying to find drug targets in B-cell non-Hodgkin lymphoma, a type of cancer that affects the body’s lymphatic system (the network of glands and lymph nodes in our bodies). She started studying cancer because “it’s one of the major health challenges facing the world today and, despite the work already done, it still impacts most of us.”

Professor Vikki Rand
B-cell non-Hodgkin lymphoma is a rare type of cancer and it’s also very aggressive. Whilst most children can be cured, Vikki says the “drugs that achieve these survival rates, and save all these children, are very toxic.” Unfortunately, treatments don’t always work for some children, and they cannot be cured.
Finding kinder treatments that don’t do as much damage to a child’s growing body is one of Vikki’s top priorities:
Cancer survivorship is a huge consideration for children with cancer, and it’s an area in which we need to improve. I believe that finding new, kinder treatments is key to this.
With her team at the National Horizons Centre at Teesside University, she has analysed the genetics of 95 cases of B-cell non-Hodgkin lymphoma to try and find specific mutations in genes associated with poor outcomes.
Finding a rogue gene called TP53
The researchers found that lots of children whose B-cell non-Hodgkin lymphoma was resistant to treatment had errors in a gene called TP53. Sometimes called ‘the guardian of the genome’, it normally helps to stop cells from multiplying too fast. Errors in the gene can help cancer cells grow and become tumours. Vikki says, “What we saw was remarkable; for the first time we could identify patients who would relapse based on the presence or absence of mutations in a specific gene.”
Whilst TP53 is known to make other cancer types more aggressive, Vikki’s project is the first to show that it affects children with B-cell non-Hodgkin Lymphoma too.
Exciting next steps
These findings suggest new opportunities to help children with B-cell non-Hodgkin lymphoma. Firstly, if patients are tested for TP53 gene errors at diagnosis, doctors will be more aware of the risk of relapse and can use stronger treatments from the start. Secondly, children who are classed as high risk, but don’t have TP53 errors, could potentially have lower doses of treatment as there is less risk of the lymphoma becoming resistant. Finally, this shows that treatments targeting the cells without normal TP53 function could work for some children with B-cell non-Hodgkin lymphoma.
As well as finding evidence that will help group patients into high-risk and low-risk, the team also found other mutations that could be potential drug targets. They are planning further research to see how each mutation is contributing to cancer and to test potential ways of stopping them.

Suzanne's research team.
Once this project is finished, Vikki and her team have plans to further this work in new projects. She says, “The exciting next step for me is looking at how to bring this knowledge to the clinic. There is a real opportunity here to improve the lives of these children, and it’s imperative that we make these changes happen.”

Ellie Ellicott is CCLG’s Research Communication Executive.
She is using her lifelong fascination with science to share the world of childhood cancer research with CCLG’s fantastic supporters. You can find Ellie on X: @EllieW_CCLG